Why Does Hydrogen Sulfide Have a Low Boiling Point
Get help with your The periodic table homework. No boiling does not remove iron from well water.
Why Does Hydrogen Have A Low Boiling Point Even Though Ir Has Dipole Dipole Forces Quora
Boiling kills microorganisms such as bacteria parasites molds and viruses.
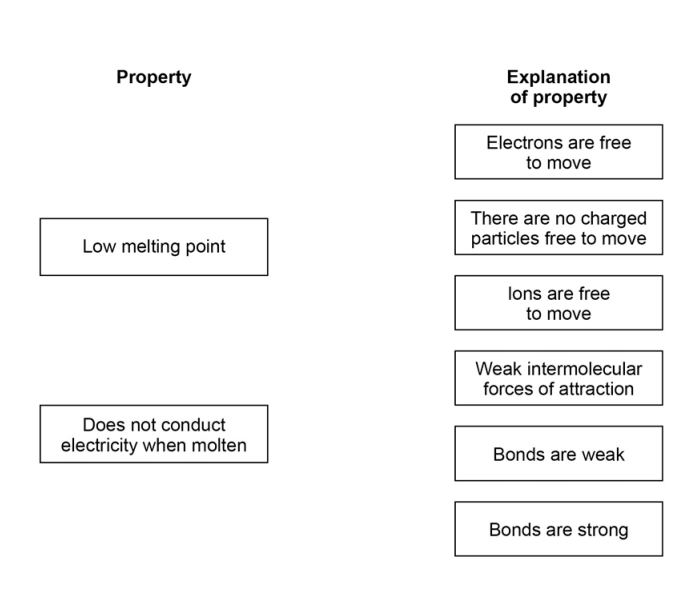
. Access the answers to hundreds of The periodic table questions that. The ordering from lowest to highest boiling point is expected to be CH 4 SiH 4 GeH 4 SnH 4. Which of the following statements is true.
For which substance will hydrogen bonding have the greater effect on the boiling point. The recommended dosage of hydrogen water does not have any federal regulation and there is no way to asses how valid the claims are. Raising the water temperature to 212 degrees Fahrenheit does not break up ferrous compounds in the water.
In which substance are the individual hydrogen bonds stronger. Explain why the hydrogen bonds in liquid HF are stronger than the corresponding intermolecular HI interactions in liquid HI. The Periodic Table Questions and Answers.
A graph of the actual boiling points of these compounds versus the period of the group 14 element shows this prediction to be correct. The boiling point of methanol is 65 degrees Celsius and the boiling point of ethanol is 78 degrees Celsius. HF or H 2 O.
HF or H 2 O. We do not know anything about the dosage or frequency of drinking with which you need to use hydrogen water to achieve health benefits says Robin Foroutan nutritionist spokesman and Academy of Nutrition. Therefore CH 4 is expected to have the lowest boiling point and SnH 4 the highest boiling point.
Boiling does not have any effect on iron because it is not a microorganism nor a living organism.
Why Is The Melting Point Of Water 0 C Higher Than That Of Hydrogen Sulphide 83 C Quora

Unit 3 Practice Questions Ppt Video Online Download
Why Does Hydrogen Fluoride Despite Having A Smaller Molecular Mass Than Hydrogen Sulphide Has A Higher Boiling Point Than Hydrogen Sulphide Quora

7 Shapes Of Molecules Intermolecular Forces Leaving Certificate Chemistry Ppt Download

Biophysical Properties Of Hydrogen Sulfide Download Table

H2o Has Higher Boiling Point Than H2s Due To
H2s Boiling Point How To Discuss

Why Does Water Show High Boiling Points As Compared To Hydrogen Sulphide Given Reason For Answer
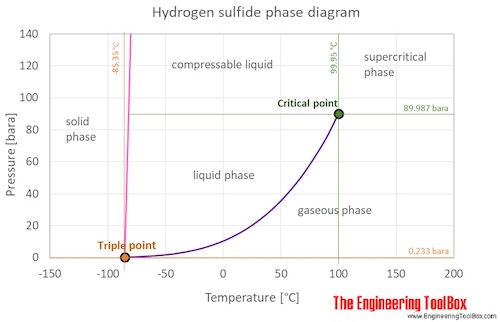
Hydrogen Sulfide Thermophysical Properties
Why Does H2o Have Low Melting And Boiling Points Than Metals Quora

Year 11 Water Practice Quiz 1 Solution 50

Solved Laboratory 4 Intermolecular Forces Useful Data Chegg Com

12 Chemistry New Book Pages 151 200 Flip Pdf Download Fliphtml5

Solved 6 Boron Trifluoride Boils At 99 9 C Even Lower Chegg Com
Why Does Hydrogen Fluoride Despite Having A Smaller Molecular Mass Than Hydrogen Sulphide Has A Higher Boiling Point Than Hydrogen Sulphide Quora

Why Does H2o Have Low Melting And Boiling Points Than Metals Quora
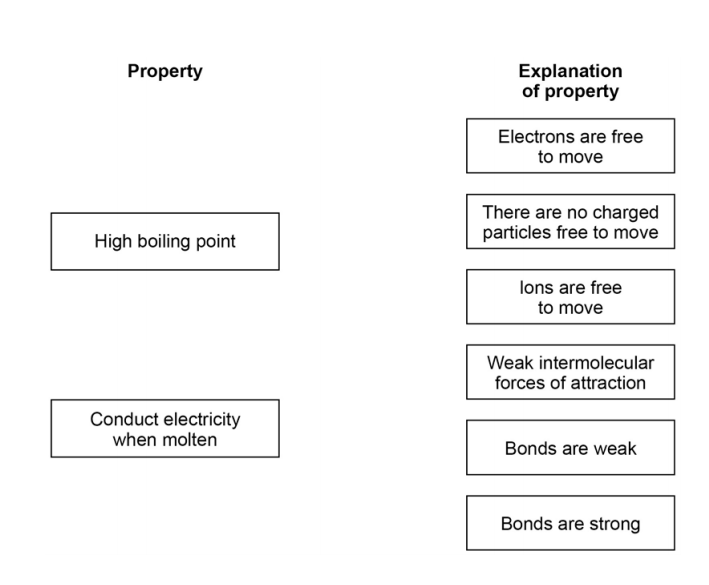
Comments
Post a Comment